Linear mixed, balanced inhibition
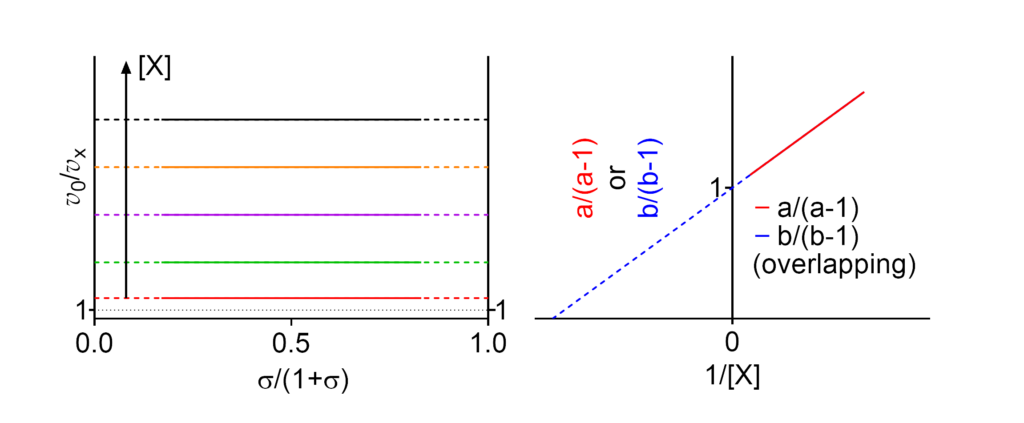
Featured examples
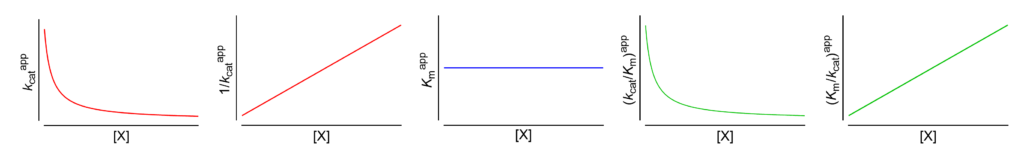
- Km⫫ The apparent Michaelis constant is independent of [X]
- V ↓ The apparent limiting rate decreases with increasing [X]
These symbols are shown only when the featured dependencies of the parameters on modifier concentration have been demonstrated by the authors.
| # | Enzyme Species | EC no. | Modifier | Substrate(1) | Name given by authors (2) | Reference(3) |
|---|---|---|---|---|---|---|
| 1 | Aminopeptidase N Homo sapiens | 3.4.11.2 | Deoxycholic acid | Leu-p-nitroanilide | Pure noncompetitive inhibition α = 1.0, KX = 0.66 mM | Nakanishi (1989) |
| 2 | Aminopeptidase N Homo sapiens | 3.4.11.2 | Chenodeoxycholic acid | Leu-p-nitroanilide | Pure noncompetitive inhibition α = 1.0, KX = 0.21 mM | Nakanishi (1989) |
| 3 | Caspase-7 Homo sapiens | 3.4.22.60 | NSC321205 | (Z-DEVD)2Rh110 (4) | Noncompetitive inhibition, Km⫫, V ↓ α = 1.01, β = 0.06, KX = 0.34 μM | Feldman (2012) |
| 4 | Caspase-7 Homo sapiens | 3.4.22.60 | NSC277584 | (Z-DEVD)2Rh110 (4) | Noncompetitive inhibition, Km⫫, V ↓ α = 0.94, β = 0.09, KX = 0.41 μM | Feldman (2012) |
| 5 | Caspase-7 Homo sapiens | 3.4.22.60 | NSC310547 | (Z-DEVD)2Rh110 (4) | Noncompetitive inhibition, Km⫫, V ↓ α = 1.04, β = 0.08, KX = 0.34 μM | Feldman (2012) |
| 6 | Caspase-9-LZ (5) Homo sapiens | 3.4.22.62 | NSC321205 | Ac-LEHD-AFC (6) | Noncompetitive inhibition, Km⫫, V ↓ α = 1.06, β = 0.08, KX = 0.08 μM | Feldman (2012) |
| 7 | Caspase-9-LZ (5) Homo sapiens | 3.4.22.62 | NSC277584 | Ac-LEHD-AFC (6) | Noncompetitive inhibition, Km⫫, V ↓ α = 0.92, β = 0.04, KX = 0.18 μM | Feldman (2012) |
| 8 | Caspase-9-LZ (5) Homo sapiens | 3.4.22.62 | NSC310547 | Ac-LEHD-AFC (6) | Noncompetitive inhibition, Km⫫, V ↓ α = 1.08, β = 0.04, KX = 0.17 μM | Feldman (2012) |
| 9 | Caspase-9 Homo sapiens | 3.4.22.62 | NSC321205 | Ac-LEHD-AFC (6) | Noncompetitive inhibition, Km⫫, V ↓ α = 1.04, β = 0.04, KX = 0.12 μM | Feldman (2012) |
| 10 | Caspase-2 Homo sapiens | 3.4.22.55 | NSC277584 | (Z-DEVD)2Rh110 (4) | Noncompetitive inhibition, Km⫫, V ↓ α = 0.98, β = 0.02, KX = 0.38 μM | Feldman (2012) |
| 11 | Caspase-2 Homo sapiens | 3.4.22.55 | NSC321206 | (Z-DEVD)2Rh110 (4) | Noncompetitive inhibition, Km⫫, V ↓ α = 1.04, β = 0.05, KX = 0.22 μM | Feldman (2012) |
| 12 | Caspase-2 Homo sapiens | 3.4.22.55 | NSC310547 | (Z-DEVD)2Rh110 (4) | Noncompetitive inhibition, Km⫫, V ↓ α = 0.97, β = 0.06, KX = 0.35 μM | Feldman (2012) |
| 13 | Memapsin 2 (7) Homo sapiens | 3.4.23.46 | L-685,458 pH = 6.5, 37°C | C100 (8) | Linear non-competitive inhibition (9) α = 0.97 ± 0.31, KX = 7.5 μM | Tian (2002) |
| 14 | Memapsin 2 (7) Homo sapiens | 3.4.23.46 | Compound 2 pH = 6.5, 22°C | C100 (8) | Linear non-competitive inhibition (9) α = 1.15 ± 0.37, KX = 0.13 μM | Tian (2002) |
| 15 | Memapsin 2 (7) Homo sapiens | 3.4.23.46 | Compound 3 pH = 6.5, 22°C | C100 (8) | Linear non-competitive inhibition (9) α = 0.67 ± 0.49, KX = 3.0 μM | Tian (2002) |
| 16 | Memapsin 2 (7) Homo sapiens | 3.4.23.46 | Compound 4 pH = 6.5, 22°C | C100 (8) | Linear non-competitive inhibition (9) α = 2.0 ± 1.4, KX = 0.3 nM | Tian (2002) |
| 17 | Glycogen phosphorylase Neurospora crassa | 2.4.1.1 | Uridine diphosphate glucose | Glycogen | Linear non-competitive inhibition (10) α = 1, KX = 2.1-3.2 mM | Gold (1974) |
(1) Always the varied substrate. In two- or more-substrate reactions the concentration(s) of the non varied substrate(s) is/are kept constant.
(2) Name of the mechanism given by the authors in the quoted reference. α, β and the inhibition/activation constants for the modifier (X), uniformly denoted KX, are the values specified by the authors. In some cases, missing parameters have been calculated from graphical or tabular data provided in the papers. In two- or more-substrate reactions, KX represents an apparent constant at given concentrations of the fixed substrates and no calculations of the intrinsic values have been attempted.
(3) Full references at the end of the page provide also the digital object identifier (doi), if available. Clicking the authors (highlighted) opens the reference in PubMed.
(4) (Z-DEVD)2Rh110 = (Cbz-Asp-Glu-Val-Asp)2 Rhodamine 110
(5) Engineered caspase-9 dimer that contains a leucine-zipper dimerization domain. The activity of native caspase-9 requires an active apoptosome complex, while caspase-9-LZ is constitutively active
(6) Ac-LEHD-AFC = Acetyl-Leu-Glu-His-Asp-7-amino-4-trifluoromethylcoumarin
(7) Memapsin 2 is the recommended name of an enzyme with 82 synonyms (BRENDA, June 2018), including β-secretase and γ-secretase. It is part of a complex membrane-bound macromolecular complex that involves presenilins 1 and 2
(8) C100 = Recombinant protein with amino acid sequence identical to the C-terminal fragment of presenilin (cleaved by memapsin 2 from the amyloid precursor protein) bearing an extra methionine residue at the N-terminus
(9) In this study Tian et al. (2002) paid particular attention to accurate determinations of Kis and Kii, the specific and the catalytic components of the mechanisms, respectively, providing errors and graphical representations. The linearity of inhibition was ascertained experimentally. Taking into account error propagation, these data have been used in this table to calculate α as Kii/Kis with its associated error that, together with graphics, was useful to identify the mechanism. The authors called all mechanisms ‘linear non-competitive inhibition’, which belong however to LMx(Sp>Ca)I, LMx(Sp<Ca)I and LMx(Sp=Ca)I. See also the tables of LMx(Sp>Ca)I and LMx(Sp<Ca)I
(10) Synthesis direction
References
- Feldman T, Kabaleeswaran V, Jang SB, Antczak C, Djaballah H, Wu H, Jiang X (2012) A class of allosteric caspase inhibitors identified by high-throughput screening. Mol Cell 47: 585-595. doi:10.1016/j.molcel.2012.06.007
- Gold MH, Farrand RJ, Livoni JP, Segel IH (1974) Neurospora crassa glycogen phosphorylase: Interconversion and kinetic properties of the “active” form. Arch Biochem Biophys 161: 515-527. doi:10.1016/0003-9861(74)90334-8
- Nakanishi M, Moriyama A, Narita Y, Sasaki M (1989) Aminopeptidase-M from human liver. II. Kinetic analysis of inhibition of the enzyme by bile acids. J Biochem 106: 826-830. doi:10.1093/oxfordjournals.jbchem.a122938
- Tian GC, Sobotka-Briner CD, Zysk J, Liu XD, Birr C, Sylvester MA, Edwards PD, Scott CD, Greenberg BD (2002) Linear non-competitive inhibition of solubilized human gamma-secretase by pepstatin a methylester, L685458, sulfonamides, and benzodiazepines. J Biol Chem 277: 31499-31505. doi:10.1074/jbc.M112328200