Linear mixed, predominantly catalytic inhibition
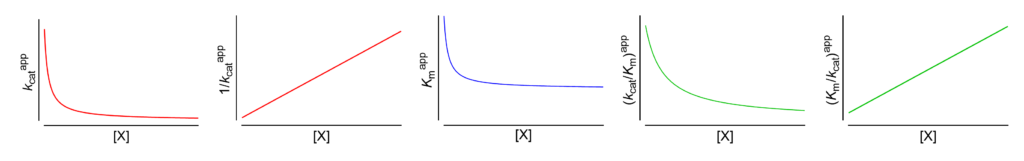
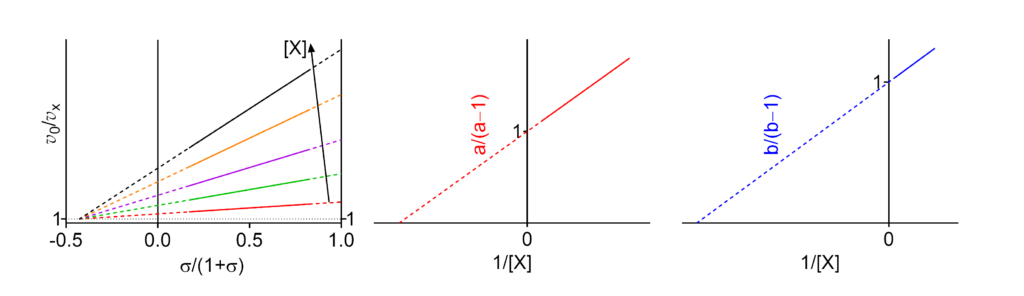
Featured examples
| # | Enzyme Species | EC no. | Modifier | Substrate(1) | Name given by authors(2) | Reference(3) |
|---|---|---|---|---|---|---|
| 1 | Aminopeptidase N Homo sapiens | 3.4.11.2 | Cholic acid | Leu-p-nitroanilide | Noncompetitive- uncompetitive linear mixed type inhibitiom α = 0.6, KX = 1.03 mM | Nakanishi (1989) |
| 2 | Memapsin 2(4) Homo sapiens | 3.4.23.46 | Compound 1 pH = 6.5, 22°C | C100(5) | Linear non-competitive inhibition(6) α = 0.54 ± 0.26, KX = 5.4 nM | Tian (2002) |
| 3 | Aldehyde oxidase Homo sapiens | 1.2.3.1 | 2,6-Dichlorophenolindophenol | Phtalazine | Mixed, predominantly uncompetitive inhibition α = 0.13, KX = 19 μM | Barr (2011) |
| 4 | Aldehyde oxidase Homo sapiens | 1.2.3.1 | Estradiol | Phtalazine | Mixed, predominantly uncompetitive inhibition α = 0.14, KX = 0.9 μM | Barr (2011) |
| 5 | Aldehyde oxidase Homo sapiens | 1.2.3.1 | Menadione | Phtalazine | Mixed, predominantly uncompetitive inhibition α = 0.16, KX = 0.75 μM | Barr (2011) |
| 6 | Aldehyde oxidase Homo sapiens | 1.2.3.1 | Ethinyl estradiol | Phtalazine | Mixed, predominantly uncompetitive inhibition α = 0.21, KX = 1.1 μM | Barr (2011) |
| 7 | Spermine synthase Bos taurus | 2.5.1.22 | 5'-methylthioadenosine (reaction product) | Spermidine | Mixed, predominantly uncompetitive inhibition α = 0.1, KX = 10 μM | Pajula (1983)(7) |
| 8 | Tyrosinase(8) Homo sapiens | 1.14.18.1 | 4-Acetylphenylthiourea | L-Dopa | Mixed, predominantly uncompetitive inhibition α = 0.41, KX = 20.2 μM | Sun (2014)(9) |
| 9 | Butane-utilizing bacterial cultures(10) | — | Butane | 1,1,1,-Trichloroethane | Mixed inhibition α = 0.69, KX = 0.52 μM | Kim (2002) |
| 10 | ABC-type quaternary amine transporter(11) Homo sapiens | 7.6.2.9 | N-(1-benzyl-4-piperidinyl)-2,4-dichlorobenzamide | γ-Aminobutyric acid | Noncompetitive inhibition α ≈ 0.09, KX ≈ 4 μM(12) | Kragholm (2013) |
| 11 | Proton-translocating NAD(P)+ transhydrogenase Escherichia coli | 7.1.1.1 | 2'-AMP | 3-Acetylpyridine adenine dinucleotide (at fixed NADPred) | Noncompetitive inhibition α = 0.5, KX = 8.0 mM | Hanson (1979) |
(1) Always the varied substrate. In two- or more-substrate reactions the concentration(s) of the non varied substrate(s) is/are kept constant.
(2) Name of the mechanism given by the authors in the quoted reference. α, β and the inhibition/activation constants for the modifier (X), uniformly denoted KX, are the values specified by the authors. In some cases, missing parameters have been calculated from graphical or tabular data provided in the papers. In two- or more-substrate reactions, KX represents an apparent constant at given concentrations of the fixed substrates and no calculations of the intrinsic values have been attempted.
(3) Full references at the end of the page provide also the digital object identifier (doi), if available. Clicking the authors (highlighted) opens the reference in PubMed.
(4) Memapsin 2 is the recommended name of an enzyme with numerous synonyms, including β-secretase and γ-secretase. It is part of a membrane-bound macromolecular complex that involves presenilins 1 and 2.
(5) C100 = Recombinant protein with amino acid sequence identical to the C-terminal fragment of presenilin (cleaved by memapsin 2 from the amyloid precursor protein) bearing an extra methionine residue at the N-terminus.
(6) In this study Tian et al. (2002) paid particular attention to accurate determinations of Kis and Kii, the specific and the catalytic components of the mechanisms, respectively, providing errors and graphical representations. The linearity of inhibition was ascertained experimentally. Taking into account error propagation, these data have been used in this table to calculate α as Kii/Kis with its associated error that, together with graphics, was useful to identify the mechanism. The authors called all mechanisms ‘linear non-competitive inhibition’, which belong however to LMx(Sp>Ca)I, LMx(Sp<Ca)I and LMx(Sp=Ca)I. See also the tables of LMx(Sp>Ca)I and LMx(Sp=Ca)I.
(7) A study dedicated to inhibition of spermine synthase (two substrates-two products reaction) by reaction products that suggests a compulsory-order mechanism in which both substrates bind the enzyme before release of products. See also the pages of LMx(Sp>Ca)I and LSpI.
(8) In this study a truncated, His-tagged form of tyrosinase, which includes the catalytic domain, was used.
(9) This paper by Sun and coworkers is a recommended reading because it is a useful tutorial in the management of enzyme-inhibition kinetic data. The authors fit the equation of the general modifier mechanism of Botts and Morales to an entire ensemble of data with a large number of experimental points. The analysis of inhibition mechanism is based on graphical representation of data and on statistical test that include the Akaike information criterion. See also results from this study under LMx(Sp>Ca)I and LSpI. Note that the authors refer to 4-acetylphenylthiourea as modifier, whereas their Figure 4c features 3-acetylphenylthiourea (the linked structure in this website shows both isomers).
(10) Aerobic cometabolism cultures of Gram-positive and Gram-negative bacteria aimed at decontaminating groundwater from chlorinated hydrocarbons. Despite the enzymes responsible for the decontaminating reactions could not be identified, the inhibition results are clear and give precious information for the optimization of bacterial growth conditions in plants of water decontamination.
(11) Transporters belong to the EC-enzyme class 7, translocases, added in 2018 to the other 6 enzyme classes. This paper by Kragholm et al. contains a kinetic study of a human ABC-type quaternary amine transporter commonly known as betaine/GABA transporter 1, a target for the treatment of epilepsy. Besides the inhibitor featured here, also tiagabine was investigated (see under HMx(Sp > Ca)I).
(12) Approximate calculations from the data in the paper.
References
- Barr JT, Jones JP (2011) Inhibition of human liver aldehyde oxidase: implications for potential drug-drug interactions. Drug Metab Disp 39: 2381-2386. doi:10.1124/dmd.111.041806
- Hanson RL (1979) The kinetic mechanism of pyridine nucleotide transhydrogenase from Escherichia coli. J Biol Chem 254: 888-893.
- Kim Y, Arp DJ, Semprini L (2002) Kinetic and inhibition studies for the aerobic cometabolism of 1,1,1-trichloroethane, 1,1-dichloroethylene, and 1,1-dichloroethane by a butane-grown mixed culture. Biotechnol Bioeng 80: 498-508. doi:10.1002/bit.10397
- Kragholm B, Kvist T, Madsen KK, Jørgensen L, Vogensen SB, Schousboe A, Clausen RP, Jensen AA, Bräuner-Osborne H (2013) Discovery of a subtype selective inhibitor of the human betaine/GABA transporter 1 (BGT-1) with a non-competitive pharmacological profile. Biochem Pharmacol 86: 521-528. doi:10.1016/j.bcp.2013.06.007
- Nakanishi M, Moriyama A, Narita Y, Sasaki M (1989) Aminopeptidase-M from human liver. II. Kinetic analysis of inhibition of the enzyme by bile acids. J Biochem 106: 826-830. doi:10.1093/oxfordjournals.jbchem.a122938
- Pajula RL (1983) Kinetic properties of spermine synthase from bovine brain. Biochem J 215: 669-676. doi:10.1042/bj2150669
- Sun W, Wendt M, Klebe G, Röhm KH (2014) On the interpretation of tyrosinase inhibition kinetics. J Enzyme Inhib Med Chem 29: 92-99. doi:10.3109/14756366.2012.755621
- Tian GC, Sobotka-Briner CD, Zysk J, Liu XD, Birr C, Sylvester MA, Edwards PD, Scott CD, Greenberg BD (2002) Linear non-competitive inhibition of solubilized human gamma-secretase by pepstatin a methylester, L685458, sulfonamides, and benzodiazepines. J Biol Chem 277: 31499-31505. doi:10.1074/jbc.M112328200