Hyperbolic mixed, balanced activation
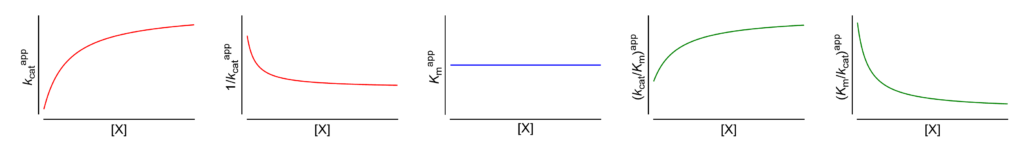
Featured examples
V ↑ (∴ kcat↑) The apparent limiting rate, and therefore the catalytic constant, increase with increasing [X]
These symbols are shown only when the featured dependencies of the parameters on modifier concentration have been demonstrated by the authors.
| # | Enzyme Species | EC no. | Modifier | Substrate(1) | Name given by authors (2) | Reference(3) |
|---|---|---|---|---|---|---|
| 1 | Sucrose α-glucosidase (4) Mus musculus (Balb c) | 3.2.1.48 | Na+ (pH = 5.0) | Sucrose | V-type activation, V↑ α = 1, β ≈ 2.6, KX not calculated | Gupta (2009) |
| 2 | Trypsin Bos taurus | 3.4.21.4 | Methylammonium 0.1 M KCl | Acetyl-Gly ethyl ester | Activation, V↑ α = 1, β ≈ 3.2, KX = 380 mM | Inagami (1964) |
| 3 | Trypsin Bos taurus | 3.4.21.4 | Ethylammonium 0.1 M KCl | Acetyl-Gly ethyl ester | Activation, V↑ α = 1, β ≈ 9.5, KX = 69 mM | Inagami (1964) |
| 4 | Trypsin Bos taurus | 3.4.21.4 | 1-Propylammonium 0.1 M KCl | Acetyl-Gly ethyl ester | Activation, V↑ α = 1, β ≈ 2.4, KX = 86 mM | Inagami (1964) |
| 5 | Trypsin Bos taurus | 3.4.21.4 | Methylguanidine | Acetyl-Gly ethyl ester | Activation, V↑ α = 1, β ≈ 7, KX = 13 mM | Inagami (1968) |
| 6 | Trypsin Bos taurus | 3.4.21.4 | Ethylguanidine | Acetyl-Gly ethyl ester | Activation, V↑ α = 1, β ≈ 2, KX = 2 mM | Inagami (1968) |
| 7 | Myeloblastin Homo sapiens | 3.4.21.76 | Chondroitin sulfate | MeO-Suc-AAPV-7-amino-4-methylcoumarylamide | Nonessential activation, V↑ α = 1, β = 5.5, KX = 1.2 mM | Früh (1996) |
| 8 | Myeloblastin Homo sapiens | 3.4.21.76 | Dermatan sulfate | MeO-Suc-AAPV-7-amino-4-methylcoumarylamide | Nonessential activation, V↑ α = 1, β = 5.3, KX = 0.13 mM | Früh (1996) |
| 9 | Myeloblastin Homo sapiens | 3.4.21.76 | GAGPS (5) | MeO-Suc-AAPV-7-amino-4-methylcoumarylamide | Nonessential activation, V↑ α = 1, β = 6.7, KX = 2.1 mM | Früh (1996) |
| 10 | Long-chain fatty acid omega-monooxygenase Homo sapiens | 1.14.13.205 | Dithiothreitol | Lauric acid | Wild type enzyme Activation, V↑ α = 1, β = 2.0 (6) | Albertolle (2017) |
| 11 | Long-chain fatty acid omega-monooxygenase Homo sapiens | 1.14.13.205 | Dithiothreitol | Lauric acid | F434S variant of the enzyme Activation, V↑ α = 1, β = 2.1 (6) | Albertolle (2017) |
| 12 | HtrA2 peptidase Homo sapiens | 3.4.21.108 | HAX-1 [Hematopoietic cell-specific protein 1 (HS1)-associated protein X-1] | Fluorescein isothiocyanate-labeled β-casein | V-system of allosteric modulation Activation: V↑, K0.5 unchanged Modifier binding cooperative (7) | Chaganti (2019) |
| 13 | Non-specific serine/ threonine protein kinase Species undeclared | 2.7.11.1 | Pyrvinium | α-Casein | Activation, V↑ (8) | Shen (2019) |
(1) Always the varied substrate. In two- or more-substrate reactions the concentration(s) of the non varied substrate(s) is/are kept constant.
(2) Name of the mechanism given by the authors in the quoted reference. α, β and the inhibition/activation constants for the modifier (X), uniformly denoted KX, are the values specified by the authors. In some cases, missing parameters have been calculated from graphical or tabular data provided in the papers. In two- or more-substrate reactions, KX represents an apparent constant at given concentrations of the fixed substrates and no calculations of the intrinsic values have been attempted.
(3) Full references at the end of the page provide also the digital object identifier (doi), if available. Clicking the authors (highlighted) opens the reference in PubMed.
(4) The mechanism is species- and pH-dependent. See also under HSpA and HMx(Sp=Ca)I.
(5) Glycosaminoglycan polysulfate: derived from chondroitin sulfate by additional sulfation (average of 3.25 sulfate groups per disaccharide unit).
(6) Calculated from Figure 2. KX could not be calculated from just one concentration of modifier (dithiothreitol = 1 mM), which is possibly a saturating concentration allowing the calculation of β as the ratio of the limiting rates in the presence and absence of modifier.
(7) A clear demonstration of hyperbolic mixed, balanced activation. In the presence of HAX-1, the limiting rate increases, K0.5 remains constant, and the specificity constant increases. In the absence of modifier the Hill coefficient is 2.4, and in its presence it is 1.9.
(8) With just one modifier concentration, the value of KX could not be measured, but the modifier mechanism is clearly hyperbolic mixed, balanced activation as deduced from the results presented in Fig. 4 and Table 1
References
- Albertolle ME, Kim D, Nagy LD, Yun C-H, Pozzi A, Savas Ü, Johnson EF, Guengerich FP (2017) Heme–thiolate sulfenylation of human cytochrome P450 4A11 functions as a redox switch for catalytic inhibition. J Biol Chem 292: 11230-11242. doi:10.1074/jbc.M117.792200
- Chaganti LK, Dutta S, Kuppili RR, Mandal M, Bose K (2019) Structural modeling and role of HAX-1 as a positive allosteric modulator of human serine protease HtrA2. Biochem J 476: 2965-2980. doi:10.1042/bcj20190569
- Früh H, Kostoulas G, Michel BA, Baici A (1996) Human myeloblastin (leukocyte proteinase 3): Reactions with substrates, inactivators and activators in comparison with leukocyte elastase. Biol Chem 377: 579-586. doi:10.1515/bchm3.1996.377.9.579
- Gupta S, Mahmood S, Mahmood A (2009) Kinetic characteristics of brush border sucrase activation by Na+ ions in mice intestine. Indian J Exp Biol 47: 811-815.
- Inagami T, Murachi T (1964) The mechanism of the specificity of trypsin catalysis. III. Activation of the catalytic site of trypsin by alkylammonium ions in the hydrolysis of acetylglycine ethyl ester. J Biol Chem 239: 1395-1401.
- Inagami T, York SS (1968) Effect of alkyl guanidines and alkyl amines on trypsin catalysis. Biochemistry 7: 4045-4052. doi:10.1021/bi00851a036
- Shen C, Li B, Astudillo L, Deutscher MP, Cobb MH, Capobianco AJ, Lee E, Robbins DJ (2019) The CK1α activator pyrvinium enhances the catalytic efficiency (kcat/Km) of CK1α. Biochemistry 58: 5102-5106. doi:10.1021/acs.biochem.9b00891