Hyperbolic mixed, balanced inhibition
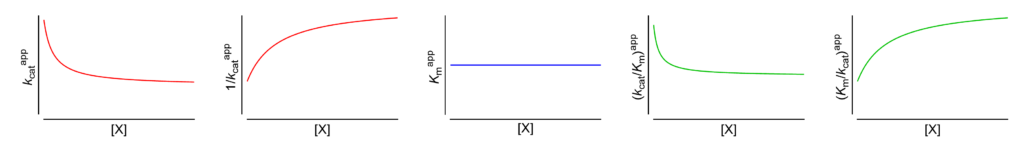
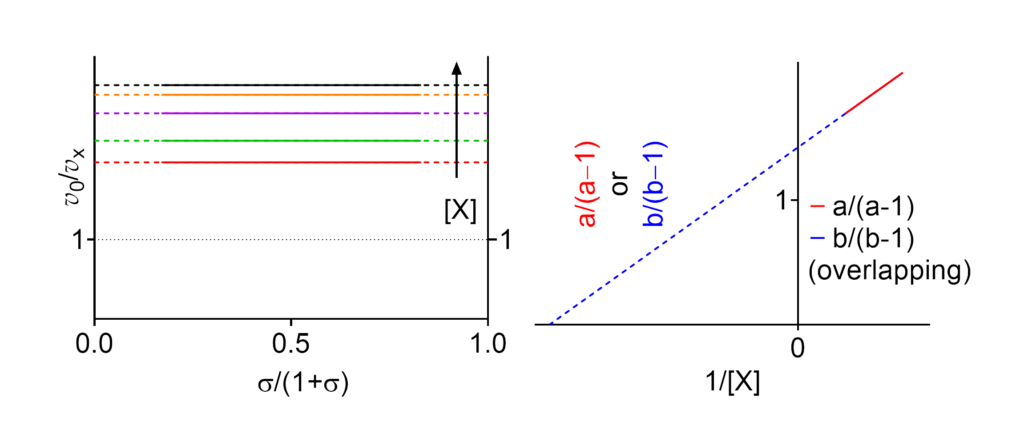
Featured examples
| # | Enzyme Species | EC no. | Modifier | Substrate(1) | Name given by authors (2) | Reference(3) |
|---|---|---|---|---|---|---|
| 1 | Leukocyte elastase Homo sapiens | 3.4.21.37 | Pentosan polysulfate | t-Butyloxycarbonyl-Ala- p-nitrophenyl ester | Hyperbolic noncompetitive inhibition α = 1, β = 0.4, KX = 0.18 μM | Baici (1981) |
| 2 | 4-Hydroxy-tetrahydro-dipicolinate synthase Escherichia coli | 4.3.3.7 | Lysine | (S)-aspartate-β-semialdehyde | Partial noncompetitive inhibition α = 1, β = 0.09, KX = 0.32 mM | Dobson (2004) |
| 3 | Plasmin Homo sapiens | 3.4.21.7 | Modified dextran RG1503 (4) | (D)-Val-Leu-Lys-p-nitroanilide | Hyperbolic noncompetitive inhibition α = 1, β = 0.62, KX = 110 nM | Ledoux (2000) |
| 4 | Plasmin Homo sapiens | 3.4.21.7 | Modified dextran RG1192 (5) | (D)-Val-Leu-Lys-p-nitroanilide | Hyperbolic noncompetitive inhibition α = 1, β = 0.2, KX = 290 nM | Ledoux (2000) |
| 5 | NADH:ubiquinone reductase (non-electrogenic) Saccharomyces cerevisiae | 1.6.5.9 | Flavone (6) | DCPIP (7) | Hyperbolic noncompetitive inhibition α = 1, β < 1, KX = 7 μM | Velázquez (2001) |
| 6 | Acetylcholinesterase Torpedo californica | 3.1.1.7 | d-Tubocurarine | 7-acetoxy-4-methylcoumarin | Nonlinear noncompetitive inhibition α = 1, β < 1, KX = 85 μM | Berman (1990) |
| 7 | Spermidine synthase Bos taurus | 2.5.1.16 | 5'-Methythioadenosine | Putrescine | Hyperbolic mixed-type inhibition Kic = Kiu = 10-20 μM (8) | Raina (1984) |
| 8 | Spermidine synthase Bos taurus | 2.5.1.16 | 5'-Methythioadenosine | Decarboxylated adenosylmethionine | Hyperbolic mixed-type inhibition Kic = Kiu = 20 μM (8) | Raina (1984) |
| 9 | Non-specific serine/ threonine protein kinase Rattus norvegicus | 2.7.11.1 | Heparin fragment (20-mer) | Peptide RRRADDSDDDDD | Hyperbolic partial non-competitive mixed type inhibition α = 1, β = 0.17, KX = 0.13 μM | O'Farrel (1999) |
| 10 | Caspase-1 Homo sapiens | 3.4.22.36 | NSC321205 | Acetyl-Tyr-Val-Ala-Asp-7-amino-4-trifluoromethylcoumarylamide | Partial noncompetitive inhibition α = 0.90, β = 0.25, KX = 1.02 μM | Feldman (2012) |
| 11 | Caspase-1 Homo sapiens | 3.4.22.36 | NSC277584 | Acetyl-Tyr-Val-Ala-Asp-7-amino-4-trifluoromethylcoumarylamide | Partial noncompetitive inhibition α = 1.02, β = 0.60, KX = 0.44 μM | Feldman (2012) |
| 12 | Caspase-1 Homo sapiens | 3.4.22.36 | NSC321206 | Acetyl-Tyr-Val-Ala-Asp-7-amino-4-trifluoromethylcoumarylamide | Partial noncompetitive inhibition α = 0.99, β = 0.34, KX = 0.80 μM | Feldman (2012) |
| 13 | Caspase-1 Homo sapiens | 3.4.22.36 | NSC310547 | Acetyl-Tyr-Val-Ala-Asp-7-amino-4-trifluoromethylcoumarylamide | Partial noncompetitive inhibition α = 1.01, β = 0.46, KX = 0.56 μM | Feldman (2012) |
| 14 | Caspase-3 Homo sapiens | 3.4.22.56 | NSC321205 | (Z-DEVD)2Rh110 (9) | Partial noncompetitive inhibition α = 1.06, β = 0.14, KX = 0.35 μM | Feldman (2012) |
| 15 | Caspase-3 Homo sapiens | 3.4.22.56 | NSC277584 | (Z-DEVD)2Rh110 (9) | Partial noncompetitive inhibition α = 0.94, β = 0.14, KX = 0.71 μM | Feldman (2012) |
| 16 | Caspase-3 Homo sapiens | 3.4.22.56 | NSC321206 | (Z-DEVD)2Rh110 (9) | Partial noncompetitive inhibition α = 0.99, β = 0.19, KX = 0.67 μM | Feldman (2012) |
| 17 | Caspase-3 Homo sapiens | 3.4.22.56 | NSC310547 | (Z-DEVD)2Rh110 (9) | Partial noncompetitive inhibition α = 1.06, β = 0.28, KX = 0.95 μM | Feldman (2012) |
| 18 | Caspase-8 Homo sapiens | 3.4.22.61 | NSC321205 | (Z-DEVD)2Rh110 (9) | Partial noncompetitive inhibition α = 0.98, β = 0.23, KX = 0.69 μM | Feldman (2012) |
| 19 | Caspase-8 Homo sapiens | 3.4.22.61 | NSC277584 | (Z-DEVD)2Rh110 (9) | Partial noncompetitive inhibition α = 1.04, β = 0.11, KX = 0.28 μM | Feldman (2012) |
| 20 | Caspase-8 Homo sapiens | 3.4.22.61 | NSC321206 | (Z-DEVD)2Rh110 (9) | Partial noncompetitive inhibition α = 1.03, β = 0.19, KX = 0.40 μM | Feldman (2012) |
| 21 | Caspase-8 Homo sapiens | 3.4.22.61 | NSC310547 | (Z-DEVD)2Rh110 (9) | Partial noncompetitive inhibition α = 1.07, β = 0.32, KX = 0.67 μM | Feldman (2012) |
| 22 | Glycogen phosphorylase Neurospora crassa | 2.4.1.1 | Glucose-6-phosphate | Glucose-1-phosphate (synthesis direction) | Partial noncompetitive inhibition α = 1, β = 0.47, KX = 2.1 mM | Gold (1974) |
| 23 | Glycogen phosphorylase Neurospora crassa | 2.4.1.1 | Glucose-6-phosphate | Glycogen (synthesis direction) | Partial noncompetitive inhibition α = 1, β = 0.42, KX = 1.6 mM | Gold (1974) |
| 24 | Glycogen phosphorylase Neurospora crassa | 2.4.1.1 | Glucose-6-phosphate | Phosphate (phosphorolysis direction) | Partial noncompetitive inhibition (10) β = 0.42-0.52, KX = 1.6-4.0 mM | Gold (1974) |
| 25 | Glycogen phosphorylase Neurospora crassa | 2.4.1.1 | Glucose-6-phosphate | Glycogen (phosphorolysis direction) | Partial noncompetitive inhibition (10) β = 0.42-0.52, KX = 1.6-4.0 mM | Gold (1974) |
| 26 | Peroxiredoxin Homo sapiens | 1.11.1.15 | 4-Methylcatechol | H2O2 | Partial mixed noncompetitive inhibition α = 1.09, β = 0.34, KX = 0.33 mM | Chow (2016) |
| 27 | Sucrose α-glucosidase (11) Mus musculus (Balb c) | 3.2.1.48 | Na+ (pH = 8.5) | Sucrose | Noncompetitive inhibition α = 1, β = 0.68, KX not calculated | Gupta (2009) |
| 28 | Caspase-7 Homo sapiens | 3.4.22.60 | NSC321206 | (Z-DEVD)2Rh110 (9) | Partial noncompetitive inhibition α = 0.84, β = 0.15, KX = 0.31 μM | Feldman (2012) |
| 29 | Caspase-2 Homo sapiens | 3.4.22.55 | NSC321205 | (Z-DEVD)2Rh110 (9) | Partial noncompetitive inhibition α = 0.96, β = 0.16, KX = 0.69 μM | Feldman (2012) |
| 30 | Leukocyte elastase Homo sapiens | 3.4.21.37 | FURA-2 | MeO-Suc-Ala-Ala-Pro-Val-p-nitroanilide | Partial noncompetitive inhibition α = 1.0, β = 0.12, KX = 0.68 mM | Tyagi (1991) |
| 31 | Receptor protein-tyrosine kinase Homo sapiens | 2.7.10.1 | RG 14467 | Peptide K1 (12) | Hyperbolic noncompetitive inhibition α ≈ 1, β ≈ 0.25, KX ≤ 30 nM | Hsu (1991) |
(1) Always the varied substrate. In two- or more-substrate reactions the concentration(s) of the non varied substrate(s) is/are kept constant.
(2) Name of the mechanism given by the authors in the quoted reference. α, β and the inhibition/activation constants for the modifier (X), uniformly denoted KX, are the values specified by the authors. In some cases, missing parameters have been calculated from graphical or tabular data provided in the papers. In two- or more-substrate reactions, KX represents an apparent constant at given concentrations of the fixed substrates and no calculations of the intrinsic values have been attempted.
(3) Full references at the end of the page provide also the digital object identifier (doi), if available. Clicking the authors (highlighted) opens the reference in PubMed.
(4) RG1503, average Mr = 47,000. The stoichiometry of binding was enzyme:inhibitor = 6:1. Tightly bound modifier.
(5) RG1192, average Mr = 140,000, The stoichiometry of binding was enzyme:inhibitor = 20:1. Tightly bound modifier.
(6) Flavone = 2-phenyl-4H-1-benzopyran-4-one.
(7) DCPIP = 2,6-dichorophenolindophenol (replaces a natural quinone for in vitro studies)
(8) Product inhibition. Hyperbolic inhibition clearly demonstrated (α = 1, β < 1 ) but the value o. β was not shown.
(9) (Z-DEVD)2Rh110 = (Cbz-Asp-Glu-Val-Asp)2 Rhodamine 110.
(10) In the phosphorolysis direction the inhibition is reported to be partial noncompetitive with respect to both phosphate and glycogen and only a global range is given for the parameters: β = 0.42-0.52, KX= 1.6-4.0 mM.
(11) The mechanism is species- and pH-dependent. See also under HSpA and HMx(Sp=Ca)A.
(12) K1 is a peptide containing the major autophosphorylation site (Tyr-1173) of the epidermal growth factor receptor. Tight-binding (quasi irreversible) slow-onset inhibition; KX estimated by the authors. Approximate values of α and β were estimated from the data in Fig. 11.
References
- Baici A, Salgam P, Fehr K, Böni A (1981) Inhibition of human elastase from polymorphonuclear leucocytes by gold sodium thiomalate and pentosan polysulfate (SP-54). Biochem Pharmacol 30: 703-708. doi:10.1016/0006-2952(81)90154-4
- Berman HA, Leonard K (1990) Ligand exclusion on acetylcholinesterase. Biochemistry 29: 10640-10649. doi:10.1021/bi00499a010
- Chow ML, Troussicot L, Martin M, Doumèche B, Guillière F, Lancelin J-M (2016) Predicting and understanding the enzymatic inhibition of human peroxiredoxin 5 by 4-substituted pyrocatechols by combining funnel metadynamics, solution NMR, and steady-state kinetics. Biochemistry 55: 3469-3480. doi:10.1021/acs.biochem.6b00367
- Dobson RCJ, Griffin MDW, Roberts SJ, Gerrard JA (2004) Dihydrodipicolinate synthase (DHDPS) from Escherichia coli displays partial mixed inhibition with respect to its first substrate, pyruvate. Biochimie 86: 311-315. doi:10.1016/j.biochi.2004.03.008
- Feldman T, Kabaleeswaran V, Jang SB, Antczak C, Djaballah H, Wu H, Jiang X (2012) A class of allosteric caspase inhibitors identified by high-throughput screening. Mol Cell 47: 585-595. doi:10.1016/j.molcel.2012.06.007
- Gold MH, Farrand RJ, Livoni JP, Segel IH (1974) Neurospora crassa glycogen phosphorylase: Interconversion and kinetic properties of the “active” form. Arch Biochem Biophys 161: 515-527. doi:10.1016/0003-9861(74)90334-8
- Gupta S, Mahmood S, Mahmood A (2009) Kinetic characteristics of brush border sucrase activation by Na+ ions in mice intestine. Indian J Exp Biol 47: 811-815.
- Hsu CY, Persons PE, Spada AP, Bednar RA, Levitzki A, Zilberstein A (1991) Kinetic analysis of the inhibition of the epidermal growth factor receptor tyrosine kinase by lavendustin-A and its analogue. J Biol Chem 266: 21105-21112
- Ledoux D, Papy-Garcia D, Escartin Q, Sagot MA, Cao Y, Barritault D, Courtois J, Hornebeck W, Caruelle JP (2000) Human plasmin enzymatic activity is inhibited by chemically modified dextrans. J Biol Chem 275: 29383-29390. doi:10.1074/jbc.M000837200
- O’Farrell F, Loog M, Janson IM, Ek P (1999) Kinetic study of the inhibition of CK2 by heparin fragments of different length. Biochim Biophys Acta 1433: 68-75. doi:10.1016/S0167-4838(99)00147-8
- Raina A, Hyvönen T, Eloranta T, Voutilainen M, Samejima K, Yamanoha B (1984) Polyamine synthesis in mammalian tissues. Isolation and characterization of spermidine synthase from bovine brain. Biochem J 219: 991-1000. doi:10.1042/bj2190991
- Tyagi SC, Simon SR (1991) Interaction of neutrophil elastase with hydrophobic polyanionic chelators. Biochem Cell Biol 69: 624-629. doi:10.1139/o91-092
- Velázquez I, Pardo JP (2001) Kinetic characterization of the rotenone-insensitive internal NADH: ubiquinone oxidoreductase of mitochondria from Saccharomyces cerevisiae. Arch Biochem Biophys 389: 7-14. doi:10.1006/abbi.2001.2293