Hyperbolic mixed, predominantly catalytic inhibition
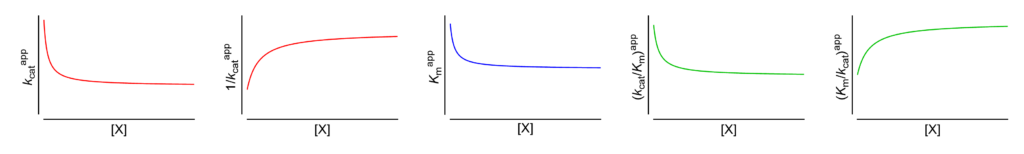
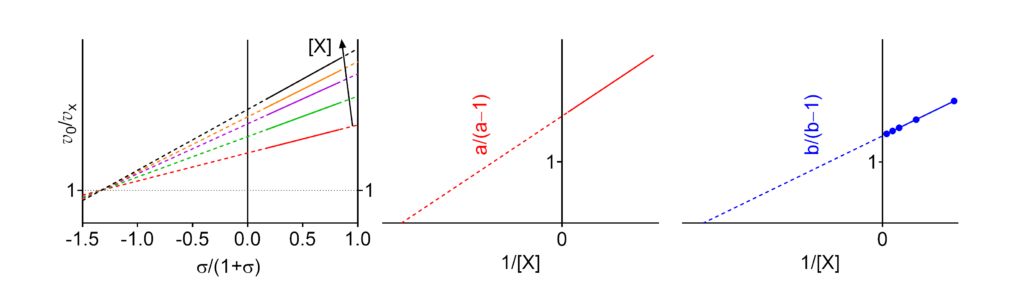
Featured examples
- Km↓ The apparent Michaelis constant decreases with increasing [X]
- V ↓ (∴kcat ↓) The apparent limiting rate, and therefore the catalytic constant, decrease with increasing [X]
These symbols are shown only when the featured dependencies of the parameters on modifier concentration have been demonstrated by the authors.
| # | Enzyme Species | EC no. | Modifier | Substrate(1) | Name given by authors (2) | Reference(3) |
|---|---|---|---|---|---|---|
| 1 | Laccase Trametes versicolor | 1.10.3.2 | Arsenite | ABTS (4) | Mixed with preference toward uncompetitive inhibition Km↓, V↓, KX = 2.3 mM | Wang (2016) |
| 2 | Glycerol kinase (5) Escherichia coli | 2.7.1.30 | IIAGlc (6) | MgATP | K-type activation & V-type inhibition, Km↓, V↓ α = 0.5, β = 0.12, KX = 1.3 μM | Pettigrew (2009) |
| 3 | Glycerol kinase (7) Escherichia coli | 2.7.1.30 | IIAGlc (6) | MgATP | K-type activation & V-type inhibition, Km↓, V↓ α = 0.25, β = 0.13, KX = 8 μM | Pettigrew (2009) |
| 4 | 'Cytochrome P450' (8) Rattus norvegicus | —(8) | p-Nitrobenzoate | Zoxazolamine | S, I-hyperbolic noncompetitive (9) | Sternson (1976) |
| 5 | Coagulation factor IXa (FIXa) Homo sapiens | 3.4.21.22 | Nitrophorin-2 | S-2765, mix (a) (11) | Hyperbolic mixed-type inhibition, Km↓, V↓ α = 0.56, β = 0.19, KX = 15.5 nM | Zhang (1998) |
| 6 | Coagulation factor IXa (FIXa) Homo sapiens | 3.4.21.22 | Nitrophorin-2 | S-2765, mix (b) (11) | Hyperbolic mixed-type inhibition, Km↓, V↓ α = 0.23, β = 0.14, KX = 16.5 nM | Zhang (1998) |
| 7 | Coagulation factor IXa (FIXa) Homo sapiens | 3.4.21.22 | Nitrophorin-2 | S-2765, mix (c) (11) | Hyperbolic mixed-type inhibition, Km↓, V↓ α = 0.12, β = 0.023, KX = 23.3 nM | Zhang (1998) |
| 8 | Coagulation factor IXa (FIXa) Homo sapiens | 3.4.21.22 | Nitrophorin-2 | S-2765, mix (d) (11) | Hyperbolic mixed-type inhibition, Km↓, V↓ α = 0.07, β = 0.035, KX = 12.6 nM | Zhang (1998) |
| 9 | Coagulation factor IXa (FIXa) Homo sapiens | 3.4.21.22 | Nitrophorin-2 | S-2765, mix (e) (11) | Hyperbolic mixed-type inhibition, Km↓, V↓ α = 0.57, β = 0.21, KX = 21.6 nM | Zhang (1998) |
| 10 | Acetylcholinesterase Electrophorus electricus | 3.1.1.7 | Compound 9 | Acetylthiocholine | Hyperbolic mixed-type inhibition α = 0.7, β = 0.08, KX = 1.6 μM | González-Tanarro (2011) |
| 11 | Acetylcholinesterase Electrophorus electricus | 3.1.1.7 | Compound 10 | Acetylthiocholine | Hyperbolic mixed-type inhibition α = 0.47, β = 0.24, KX = 2.1 μM | González-Tanarro (2011) |
| 12 | Acetylcholinesterase Electrophorus electricus | 3.1.1.7 | Compound 39 | Acetylthiocholine | Mixed-type inhibition with pronounced uncompetitive character α = 0.33, β = 0.05, KX = 0.66 μM | Pietsch (2005) |
| 13 | Acetylcholinesterase Electrophorus electricus | 3.1.1.7 | Compound 40 | Acetylthiocholine | Mixed-type inhibition with pronounced uncompetitive character α = 0.37, β = 0.08, KX = 1.7 μM | Pietsch (2005) |
| 14 | β-Lactamase Pseudomonas aeruginosa | 3.5.2.6 | Cherry-NbVIM_38 (12) | Cephalothin (13) | Mixed, predominantly uncompetitive inhibition α = 0.6, β = 0.4, KX = 9 μM | Sohier (2013) |
| 15 | Tyrosinase Agaricus bisporus | 1.14.18.1 | Chloride anion, Cl– | L-Dopa | Reversible partial hyperbolic uncompetitive (14) | Park (2005) |
| 16 | Peroxidase Armoracia rusticana | 1.11.1.7 | Melamine | o-Phenylenediamine (15) pH = 4.3 | Noncompetitive inhibition Km↓, V↓ | Karaseva (2003) |
| 17 | Thrombin Homo sapiens | 3.4.21.5 | p -Mercaptobenzoic acid-coated gold nanoparticles | H-D-Phe-Pip-Arg- p -nitroanilide | Hyperbolic mixed inhibition with predominantly uncompetitive character α = 0.43, β = 0.33, KX = 21 nM | Lira (2018) |
| 18 | Dipeptidyl-peptidase I (monomer) Homo sapiens | 3.4.14.1 | 3'-Nitrophthalanilic acid | H-Gly-Phe-7-amino-4-methylcoumarylamide | Hyperbolic mixed inhibition α = 0.40, β = 0.26, KX = 560 μM | Rebernik (2019) |
| 19 | Purine nucleosidase Trypanosoma vivax | 3.2.2.2 | Dromedary antibody variable domain fragment (VHH 1589) | p-nitrophenyl riboside | Hyperbolic mixed inhibition α = 0.76, β = 0.46, KX = 67 nM | Barlow (2009) |
(1) Always the varied substrate. In two- or more-substrate reactions the concentration(s) of the non varied substrate(s) is/are kept constant.
(2) Name of the mechanism given by the authors in the quoted reference. α, β and the inhibition/activation constants for the modifier (X), uniformly denoted KX, are the values specified by the authors. In some cases, missing parameters have been calculated from graphical or tabular data provided in the papers. In two- or more-substrate reactions, KX represents an apparent constant at given concentrations of the fixed substrates and no calculations of the intrinsic values have been attempted.
(3) Full references at the end of the page provide also the digital object identifier (doi), if available. Clicking the authors (highlighted) opens the reference in PubMed.
(4) ABTS = 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid).
(5) With the amino acid substitution E478C.
(6) IIAGlc Glucose-specific phosphocarrier protein of the phosphoenolpyruvate:glycose phosphotransferase.
(7) With the amino acid substitution E478C, T428V and R429N.
(8) Enzyme activity in rat liver microsomes called ‘Zoxazolamine oxidase, possibly the Unspecific monooxygenase, EC 1.14.14.1. Zoxazolamine = 5-Chloro-1,3-benzoxazol-2-amine, a muscle relaxant now dismissed due to hepatotoxycity.
(9) The mechanism can be clearly diagnosed from Figure 1. However, a very personal definition of the parameters in Table I and just straight lines shown without experimental points in Figure 1A hamper the calculation of kinetic parameters.
(10) Nitrophorin-2 = ferriheme protein from the insect Rhodnius prolixus.
(11) S-2765 = N-α-benzyloxycarbonyl-D-arginyl-glycyl-arginyl-p-nitroanilide was the substrate for monitoring the generation of FXa from FX after activation by FIXa. The inhibition parameters were measured with different reaction mixtures that included, besides catalyst, substrate, inhibitor, an artificial membrane surface consisting of a mixture of L-α-phosphatidyl-L-serine (PS) and L-α-dioleoylphosphatidylcholine (PC), and FVIIIa. Either the surface or FVIIIa or both together were necessary for the inhibitory action. In all of following combinations the mechanism HMx(Sp<Ca)I was observed:
- mix (a) = PS/PC alone: FIXa (10 nM), PS/PC (20 μM), FX (10-300 nM)
- mix (b) = PS/PC + FVIIIa: FIXa (0.25 nM), PS/PC (0.5 μM), FVIIIa (15 U/mL), FX (5-150 nM)
- mix (c) = platelets alone: FIXa (10 nM), platelets (1 × 108/mL), FX (10-300 nM)
- mix (d) = platelets + FVIIIa: FIXa (0.25 nM), platelets (5 × 107/mL), FVIIIa (15 U/mL), FX (5-150 nM)
- mix (e) = FVIIIa alone: FIXa (10 nM), FVIIIa (30 U/mL), FX (10-400 nM)
(12) Cherry-NbVIM_38 = single-domain antibody fragment (camelid nanobody) against the clinically-relevant Verona integron-encoded metallo-β-lactamase. In addition to the inhibition by the nanobody, Sohier et al. (2013) also describe substrate inhibition due to compulsory addition of a second substrate molecule to the ESX complex: ESX + S ⇄ SESX.
(13) A first-generation cephalosporin antibiotic.
(14) According to Fig. 4B in (Park, 2005), the mechanism described is HMx(Sp<Ca)I. The confusion about uncompetitive interactions in this and other publications is due to misinterpretation of the basic mechanism as discussed elsewhere (pp. 150-153 and 273-276).
(15) At pH = 7.4 the mechanism changes from inhibition to activation with mechanism HCaA.
References
- Barlow JN, Conrath K, Steyaert J (2009) Substrate-dependent modulation of enzyme activity by allosteric effector antibodies. Biochimica et Biophysica Acta – Proteins and Proteomics 1794: 1259-1268. doi:10.1016/j.bbapap.2009.03.019
- González-Tanarro CM, Gütschow M (2011) Hyperbolic mixed-type inhibition of acetylcholinesterase by tetracyclic thienopyrimidines. J Enzyme Inhib Med Chem 26: 350-358. doi:10.3109/14756366.2010.504674
- Karaseva EI, Naumchik IV, Metelitza DI (2003) Noncompetitive activation of the peroxidase-catalyzed oxidation of o-phenylenediamine by melamine (in Russian). Bioorganicheskaya Khimiya 29: 49-56. doi:10.1023/A:1022278402568
- Lira AL, Ferreira RS, Torquato RJS, Oliva MLV, Schuck P, Sousa AA (2018) Allosteric inhibition of α-thrombin enzymatic activity with ultrasmall gold nanoparticles. Nanoscale Adv. doi:10.1039/C8NA00081F
- Park YD, Kim SY, Lyou YJ, Lee JY, Yang JM (2005) A new type of uncompetitive inhibition of tyrosinase induced by Cl- binding. Biochimie 87: 931-937. doi:10.1016/j.biochi.2005.06.006
- Pettigrew DW (2009) Amino acid substitutions in the sugar kinase/hsp70/actin superfamily conserved ATPase core of E. coli glycerol kinase modulate allosteric ligand affinity but do not alter allosteric coupling. Arch Biochem Biophys 481: 151-156. doi:10.1016/j.abb.2008.11.020
- Pietsch M, Gütschow M (2005) Synthesis of tricyclic 1,3-oxazin-4-ones and kinetic analysis of cholesterol esterase and acetylcholinesterase inhibition. J Med Chem 48: 8270-8288. doi:10.1021/jm0508639
- Rebernik M, Snoj T, Klemenčič M, Novinec M (2019) Interplay between tetrameric structure, enzymatic activity and allosteric regulation of human dipeptidyl-peptidase I. Arch Biochem Biophys 675: 108121. doi:10.1016/j.abb.2019.108121
- Sohier JS, Laurent C, Chevigné A, Pardon E, Srinivasan V, Wernery U, Lassaux P, Steyaert J, Galleni M (2013) Allosteric inhibition of VIM metallo-b-lactamases by a camelid nanobody. Biochem J 450: 477-486. doi:10.1042/bj20121305
- Sternson LA, Gammans RE (1976) Effect of aromatic nitro compounds on oxidative metabolism by cytochrome P-450 dependent enzymes. J Med Chem 19: 174-177. doi:10.1021/jm00223a034
- Wang T, Milton RD, Abdellaoui S, Hickey DP, Minteer SD (2016) Laccase inhibition by arsenite/arsenate: Determination of inhibition mechanism and preliminary application to a self-powered biosensor. Anal Chem 88: 3243-3248. doi:10.1021/acs.analchem.5b04651
- Zhang Y, Ribeiro JMC, Guimarães JA, Walsh PN (1998) Nitrophorin-2, a novel mixed-type reversible specific inhibitor of the intrinsic factor-X activating complex. Biochemistry 37: 10681-10690. doi:10.1021/bi973050y