Linear catalytic inhibition
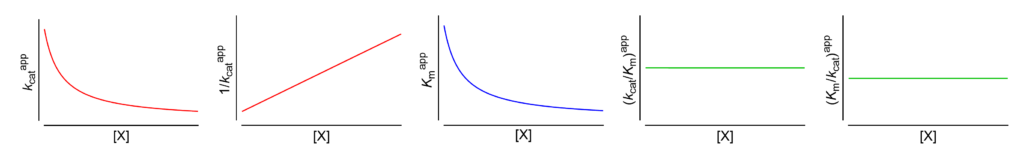
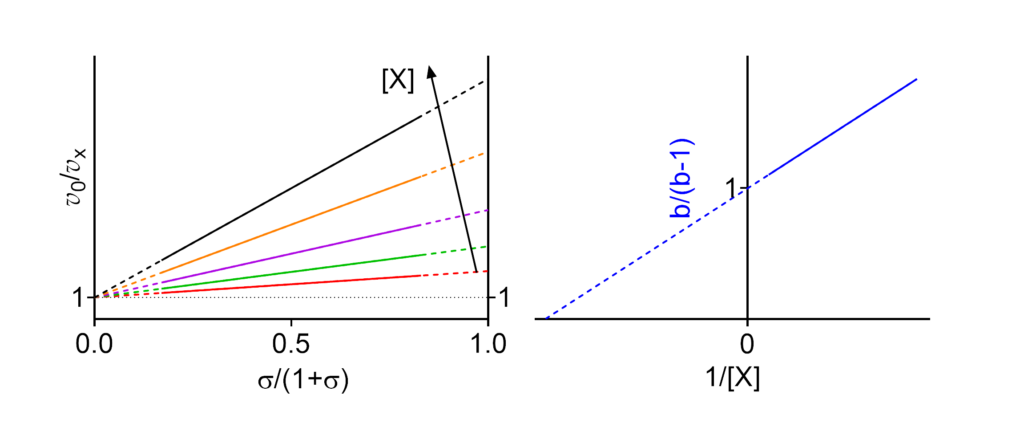
Featured examples
| # | Enzyme Species | EC no. | Modifier | Substrate(1) | Name given by authors(2) | Reference (3) |
|---|---|---|---|---|---|---|
| 1 | Catechol 1,2-dioxygenase Acinetobacter baylyi | 1.13.11.4 (4) | 4-Chlorocatechol | 4-Chloroaniline | Uncompetitive inhibition KX = 115 μM | Hongsawat (2011) |
| 2 | 3-Oxo-5alpha-steroid 4-dehydrogenase (NADP+) Rattus norvegicus | 1.3.1.22 | Aryl steroid 1 | Testosterone | Linear uncompetitive inhibition KX = 88 nM | Brandt (1990) (5) |
| 3 | 3-Oxo-5alpha-steroid 4-dehydrogenase (NADP+) Rattus norvegicus | 1.3.1.22 | Aryl steroid 1 | NADPred | Linear uncompetitive inhibition KX = 172 nM | Brandt (1990) (5) |
| 4 | 3-Oxo-5alpha-steroid 4-dehydrogenase (NADP+) Rattus norvegicus | 1.3.1.22 | Aryl steroid 2 | Testosterone | Linear uncompetitive inhibition KX = 53 nM | Brandt (1990) (5) |
| 5 | 3-Oxo-5alpha-steroid 4-dehydrogenase (NADP+) Rattus norvegicus | 1.3.1.22 | Aryl steroid 2 | NADPred | Linear uncompetitive inhibition KX = 111 nM | Brandt (1990) (5) |
| 6 | Inositol-phosphate phosphatase Bos taurus | 3.1.3.25 | Li+ | Racemic myo-inositol 1-phosphate | Linear uncompetitive inhibition (6) KX = 11.1 mM | Leech (1993) |
| 7 | Inositol-phosphate phosphatase Bos taurus | 3.1.3.25 | Li+ | myo-Inositol 4-phosphate | Linear uncompetitive inhibition (6) KX = 0.11 mM | Leech (1993) |
| 8 | Inositol-phosphate phosphatase Bos taurus | 3.1.3.25 | Li+ | Adenosine-2'-phosphate | Linear uncompetitive inhibition (6) KX = 1.52 mM | Leech (1993) |
| 9 | Alkaline phosphatase Homo sapiens | 3.1.3.1 | L-Tryptophan | Phenyl phosphate | Uncompetitive inhibition KX = 0.6 mM (37°C) | Lin (1971) |
| 10 | Gamma-glutamyltransferase Homo sapiens | 2.3.2.2 | OU749 | L-glutamic acid-γ-4-nitroanilide Transpeptidase reaction (7) | Uncompetitive inhibition KX = 73.8 μM | King (2009) |
| 11 | Gamma-glutamyltransferase Homo sapiens | 2.3.2.2 | OU749 | D-glutamic acid-γ-4-nitroanilide Hydrolysis reaction | Uncompetitive inhibition KX = 73 μM | Wickham (2012) |
| 12 | Gamma-glutamyltransferase Homo sapiens | 2.3.2.2 | Sodium benzosulfonamide | L-glutamic acid-γ-4-nitroanilide Transpeptidase reaction (7) | Uncompetitive inhibition KX = 28.8 mM | Wickham (2012) |
| 13 | Gamma-glutamyltransferase Homo sapiens | 2.3.2.2 | Sodium benzosulfonamide | D-glutamic acid-γ-4-nitroanilide Hydrolysis reaction | Uncompetitive inhibition KX = 160 mM | Wickham (2012) |
| 14 | Tyrosinase Agaricus bisporus | 1.14.18.1 | [1,4’] Bipiperidinyl-1’-yl-naphthan-2-yl- methanone | 4-[(4-methylbenzo)azo]-1,2- benzendiol | Uncompetitive inhibition KX = 5.9 μM | Karbassi (2004) |
| 15 | beta-Galactosidase Escherichia coli | 3.2.1.23 | Glycofullerene 14 (8) | p-Nitrophenyl β-D-Galactopyranoside | Uncompetitive inhibition KX = 3.2 μM | Abellán Flos (2016) |
(1) Always the varied substrate. In two- or more-substrate reactions the concentration(s) of the non varied substrate(s) is/are kept constant.
(2) Name of the mechanism given by the authors in the quoted reference. α, β and the inhibition/activation constants for the modifier (X), uniformly denoted KX, are the values specified by the authors. In some cases, missing parameters have been calculated from graphical or tabular data provided in the papers. In two- or more-substrate reactions, KX represents an apparent constant at given concentrations of the fixed substrates and no calculations of the intrinsic values have been attempted.
(3) Full references at the end of the page provide also the digital object identifier (doi), if available. Clicking the authors (highlighted) opens the reference in PubMed.
(4) And related enzymes in the bacterial culture.
(5) Measurements performed with rat liver microsomes. Double-inhibition analyses revealed cooperative binding between NADPox and aryl steroid 1 or aryl steroid 2.
(6) Up to a concentration of 1.1 mM.
(7) Transpeptidation reaction of gamma-glutamyltransferase: (5-L-glutamyl)-peptide + amino acid = peptide + 5-L-glutamyl amino acid. See also LSpI.
(8) Complex structure: see Abellán Flos et al. (2016), Supplementary Information, p. 27.
References
- Abellán Flos M, García Moreno MI, Ortiz Mellet C, García Fernández JM, Nierengarten J-F, Vincent SP (2016) Potent glycosidase inhibition with heterovalent fullerenes: Unveiling the binding modes triggering multivalent inhibition. Chem Eur J 22: 11450-11460. doi:10.1002/chem.201601673
- Brandt M, Greway AT, Holt DA, Metcalf BW, Levy MA (1990) Studies on the mechanism of steroid 5-a-reductase inhibition by 3-carboxy A-ring aryl steroids. J Steroid Biochem Mol Biol 37: 575-579. doi:10.1016/0960-0760(90)90403-8
- Hongsawat P, Vangnai AS (2011) Biodegradation pathways of chloroanilines by Acinetobacter baylyi strain GFJ2. J Hazard Mater 186: 1300-1307. doi:10.1016/j.jhazmat.2010.12.002
- Karbassi F, Saboury AA, Hassan Khan MT, Choudhary MI, Saifi ZS (2004) Mushroom tyrosinase inhibition by two potent uncompetitive inhibitors. J Enzyme Inhib Med Chem 19: 349-353. doi:10.1080/14756360409162449
- King JB, West MB, Cook PF, Hanigan MH (2009) A novel, species-specific class of uncompetitive inhibitors of gamma-glutamyl transpeptidase. J Biol Chem 284: 9059-9065. doi:10.1074/jbc.M809608200
- Leech AP, Baker GR, Shute JK, Cohen MA, Gani D (1993) Chemical and kinetic mechanism of the inositol monophosphatase reaction and its inhibition by Li+. Eur J Biochem 212: 693-704. doi:10.1111/j.1432-1033.1993.tb17707.x
- Lin CW, Sie HG, Fishman WH (1971) L-Tryptophan. A non-allosteric organ-specific uncompetitive inhibitor of human placental alkaline phosphatase. Biochem J 124: 509-516. doi:10.1042/bj1240509
- Wickham S, Regan N, West MB, Kumar VP, Thai J, Li PK, Cook PF, Hanigan MH (2012) Divergent effects of compounds on the hydrolysis and transpeptidation reactions of γ-glutamyl transpeptidase. J Enzyme Inhib Med Chem 27: 476-489. doi:10.3109/14756366.2011.597748